Analysis of enantiomers
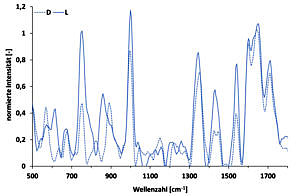
Enantiomers are optically active molecule pairs that behave like image and mirror images and have identical physicochemical properties. Only their racemate differs from the respective enantiomer. This group of molecules is particularly important in pharmacological research because most biomolecules, such as receptors, also have chiral properties, such as enantioselectivity. This leads to the fact that either only one of the two enantiomers is bioactive, or both can cause different effects in the human body. The latter case was tragically demonstrated in the 1960s with thalidomide (“thalidomide scandal”). Here D-thalidomide has a calming effect, while L-thalidomide has a teratogenic effect. The working group is therefore investigating a number of pharmaceutically relevant enantiomers for their “enantioselective” interactions. These include e.g. serine (possibly antidepressant effects), ibuprofen (antipyretic, analgesic), lysine (accelerating), tryptophan, mannitol, etc.. We investigate the formation of diastereomers and various chiral agents as well as the concentration-dependent interactions between the D- and L-enantiomers. In addition, it is now also possible to distinguish pure enantiomers using the phase-shifted Raman spectroscopy. This enables the improvement of screening processes for chiral agents and real-time tracking of enantiose separation.
